Introduction
Multiple Myeloma (MM) remains incurable despite advances in treatment. Model-based approaches based on tumor dynamics have successfully been used to optimize drug-development in oncology. Model-derived tumor dynamics also called tumor growth inhibition (TGI) metrics capture the treatment effect as tumor biomarkers and are predictive of the overall survival (OS) benefit, based on TGI-OS models, this mathematical framework has been shown to be drug-independent in most cases. M-protein is measured longitudinally during clinical trials, it reflects tumor burden and is one of the criteria used to assess clinical response according to the International Myeloma Working Group Uniform Response Criteria. A drug-independent link between M-protein dynamic metrics and OS was previously established and confirmed for refractory MM patients. The primary endpoint in MM clinical trials is usually progression free survival (PFS). New immunotherapies are currently in development and there is a need for early and robust drug development decision making. Therefore there is strong interest to expand this modeling framework to leverage M-protein dynamics to predict PFS outcomes.
Methods
CoMMpass (https://www.themmrf.org/finding-a-cure/personalized-treatment-approaches/) is a prospective, longitudinal, observational study in newly diagnosed symptomatic patients with multiple myeloma. It represents a unique source of clinical data to investigate the link between M-protein dynamic and survival outcomes across lines of therapy and treatments.
Individual M-protein and PFS Data from 804 patients within the CoMMpass study were used. Given patients are enrolled at their initial treatment regimen and observed over 8 years, they can receive several lines of therapy. Due to the characteristics of the modeling framework, allowing to analyze only one line of therapy for each patient, the latest line of therapy for each subject was selected for which they had at least 2 M-protein assessments available. M-protein dynamic metrics (M-protein ratio to baseline at different time points, time to growth, growth rate, shrinkage rate) derived using an empirical bi-exponential model, 42 prognostic factors and potential treatment effects were first tested in a univariate analysis using a Cox proportional hazards regression model. Statistically significant covariates in the univariate analysis (p<0.05) were included in a full multivariate parametric distribution survival model. A backward deletion stepwise procedure was performed (p<0.01) to retain covariates in the final PFS model. Model qualification was performed using posterior predictive checks based on Kaplan Meier plots.
Results
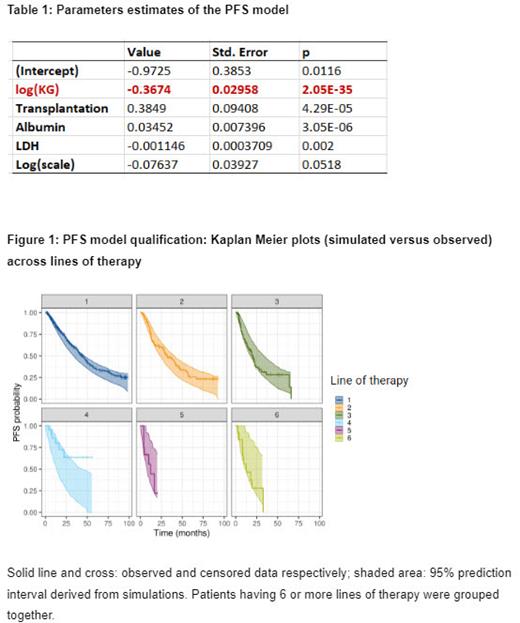
Among 804 patients, 490 patients received only 1 line of therapy (LoT), 157 received 2 LoTs, 93 received 3 LoTs, 31 received 4 LoTs,18 received 5 LoTs, 10 received 6 LoTs, finally 7 patients got up to 7 to 11 LoTs. PFS distribution followed a log-normal distribution. Table 1 shows the parameter estimates of the PFS model. Among all tested M-protein dynamic metrics and prognostic factors, the logarithmic value of the growth rate (log(KG)), in red in Table 1, was found to be the best predictor of PFS. Patients with slower growth rate, who received a transplantation, with higher albumin and lower lactate dehydrogenase values had longer PFS. Those findings are consistent with previous modeling works conducted in MM. Also, the PFS model was confirmed to be drug-independent. As shown in Figure 1, prediction intervals derived from model-based simulations well captured observed PFS data for each line of treatment although the line of therapy is not included as a predictor in the PFS model. Similar results were obtained across treatments. In a next step, external validations assessing the model performance to reproduce Phase 3 clinical trial outcomes will be performed.
Conclusions
A model linking M-protein dynamic to PFS in MM was developed across a wide range of lines of therapy and treatments. The model can support drug development in MM: enabling early predictions of clinical trial outcomes and inform study design e.g. by simulating the probability of success of upcoming pivotal clinical trials.
Disclosures
Chanu:F. Hoffmann-La Roche Ltd / Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Balasubramanian:F. Hoffmann-La Roche/Genentech, Inc.: Current Employment; AbbVie: Current equity holder in private company. Samineni:F. Hoffmann-La Roche/Genentech, Inc.: Current equity holder in publicly-traded company. Susilo:F. Hoffmann-La Roche/Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Onishi:F. Hoffmann-La Roche/Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Castro:F. Hoffmann-La Roche/Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Breuleux:F. Hoffmann-La Roche/Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Jin:F. Hoffmann-La Roche Ltd / Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Li:F. Hoffmann-La Roche Ltd / Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Bruno:F. Hoffmann-La Roche/Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal